Why join?
Health systems continue to evolve while evidence expectations, assessment methods, and the roles of national bodies and cross-border collaborations develop with them. Access teams must handle the increase in complexity both on a national and international level.
How does the access professional keep up with these developments, and where do you go to check your understanding of a particular requirement? Posts on social media, conference presentations, HTA bodies’ newsletters? There are a lot of good news sources out there, but you have to be vigilant not to miss anything, and then do you understand the context?
LiSAH brings together a range of structured resources to keep you up to date and interpret developments in the context of current practice. We provide resources to help you carry out your role and support your growth as an access professional.
What do we provide?
- We have an international access News service, focusing on changes to process and policy developments.
- We link the news to descriptions of the processes themselves - and much else besides - in our Briefings.
- Lisa, our AI-enabled assistant, is trained on our own extensive material and a broad range of validated external sources to ensure you receive the most reliable guidance when you want a quick answer to a thorny question.
- We set out an extensive set of Learning resources, including professional societies that you may want to join, courses you may find helpful, webinars and podcasts dedicated to access topics, journals, and books.
- We monitor events of interest to the access professional and allow you to identify relevant conferences, workshops and training sessions on our interactive Events calendar.
- When you need to call on the services of a specialist we have a Directory of organisations which you can filter to find those of particular relevance. As with the Events, inclusion in the Directory is free of charge so you can be sure you have the widest choice available.
Who is LiSAH for?
We have designed our service for access professionals working in Life Sciences product manufacturing and we focus on the pharmaceutical and allied sectors, including the biotechnology and advanced therapy medicinal segments.
We also recognise that there are many with a professional or personal interest in how medicines are made available for use by patients, for example:
-
Colleagues with other responsibilities in the Life Sciences sector such as in Health Economics and Outcomes Research (HEOR), Research and Development, Medical Affairs, Commercial or General Management;
-
Those who provide consultancy or other specialist services to the access function;
-
Those who represent patient organisations who want to understand how the medicines that their members depend on are approved for use in their healthcare system;
-
Employees of health authorities who are expert in their own system but who may want to check how another country addresses a particular aspect of the access process;
-
Academics who want a practical perspective on the access role to complement their research activity.
While there are aspects of our service such as health policy and health economics that we feel are of value to the medical technology sector, we recognise that we do not address the specifics of the access process for this segment. If you work in this sector and would like us to develop dedicated materials, please get in touch.
What is available for different LiSAH users?
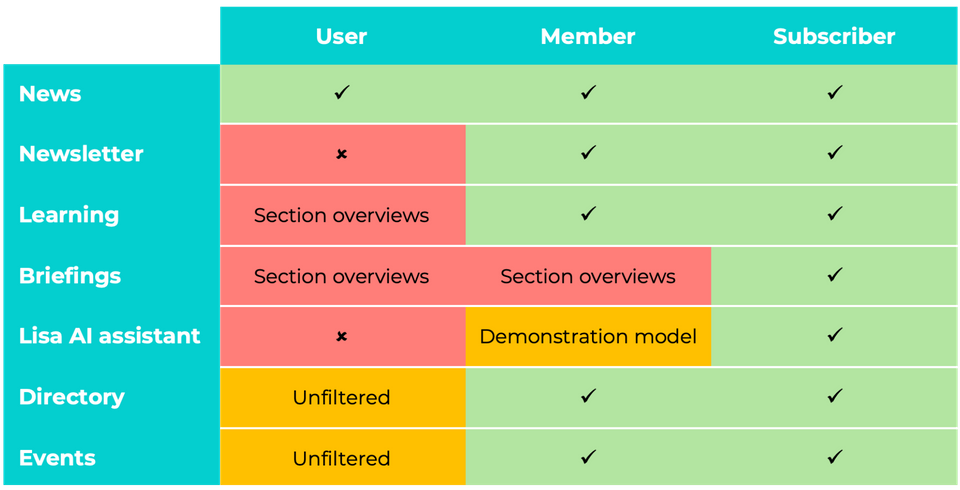
What is included?
User
- You are welcome to visit the site for access News but you do not receive the Newsletter.
- You can see what is covered in the Briefings section, but you cannot read the articles.
- You do not have access to Lisa, our AI-enabled assistant.
- You can see what is available to Members or Subscribers in the Learning section, but you cannot access the content.
- The organisations listed in our Directory are visible, but you cannot search or filter them to find those offering particular services, or with a specific geographic expertise.
- The Events calendar is also accessible but you cannot filter for conferences or trainings on particular topics or other criteria.
Member
- In addition to being able to read the access News you receive the Newsletter.
- You can see what is covered in the Briefings section, but you cannot read the articles.
- You have access to a demonstration version of Lisa, our AI-enabled assistant.
- You can read the full contents of the Learning section.
- You can search and filter the organisations listed in our Directory to find those offering particular services, or with a specific geographic expertise.
- You can also filter the Events calendar for types of events on chosen topics or other criteria.
Click here for more information.
Subscriber
- In addition to being able to read the access News you receive the Newsletter.
- You have full access to the articles in the Briefings section.
- You have access to the full version of Lisa, our AI-enabled assistant.
- You can read the full contents of the Learning section.
- You can search and filter the organisations listed in our Directory to find those offering particular services, or with a specific geographic expertise.
- You can also filter the Events calendar for types of events on chosen topics or other criteria.
Click here for more information.
